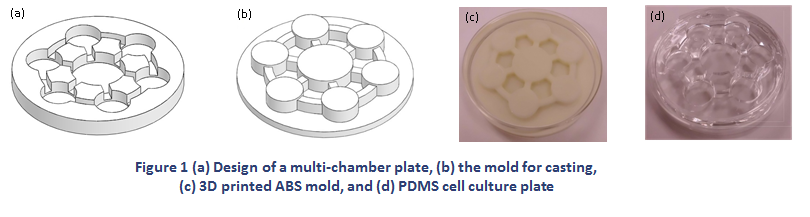
A multi-chamber cell culture plate was fabricated by casting polydimethylsiloxane (PDMS) in a 3D printed mold to study the target and off-target toxicities of a therapeutic agent on multiple organs. The plate was designed to allow seven chambers, each housing cells derived from an organ, to share the medium through inter-connecting channels. The multi-chamber plate formulates an efficient co-culture model where the therapeutic effect of a drug and unintended toxicities on multiple organs can be evaluated simultaneously. The mold used in casting was generated by selectively layering acrylonitrile-butadiene-styrene (ABS) material using a 3D printer. Although ABS is a suitable material for cell culture, it may deform or even break during the curing process of PDMS in a heated oven. As the ability to reuse the 3D printed mold is of critical importance to expedite the prototyping process, we developed a theoretical model to analyze heat transfer in the PDMS plate and the ABS mold when they were heated in the oven. The thermal conductivity of the mold was determined by the theory of heat transfer in porous media. The model was then used to predict temperature distribution in PDMS and the ABS mold.
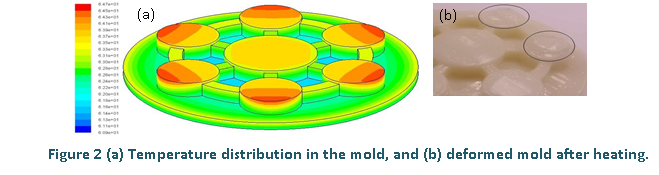
The steady state temperature on the surface of the mold is shown in Fig. 2a. It can be seen that the maximum temperature of the ABS mold occurs at the outer corner of each cylindrical column. The locations correspond to those observed on the deformed mold due to overheating as shown in Fig. 2b.
Human primary hepatocytes cultured in the produced PDMS culture plates maintained typical morphology over a 72-hour period. The viability of cells cultured in the PDMS plates was comparable to that of HPH cultured in standard collagen-coated 12-well plates. Further, a prototypical MTT assay demonstrated no difference in cell viability between HPH cultured in the two plates after 72 hours. These results indicate that the cast PDMS plates are suitable for culture and growth of cell lines for in vitro experimentation.