Nanoparticles have found important applications in novel hyperthermia treatment of cancers due to their ability to generate impressive levels of heat when excited by an external magnetic field or laser irradiation [1]. Nanoparticles can be delivered to tumors by continuous injection of nanofluid under positive pressure gradient [2]. The transport of nanoparticles in biological tissues during an infusion is a complex process that involves nanofluid flow through deformable tissues, advection of particles in the porous structures, particle binding to the cellular structure, and interactions among the particles. The particle distribution after infusion is central to the therapeutic outcome. However, quantitative characterization of the nanoparticle distribution in tissue after infusion is very limited due to the opaque nature of the tissue. A multi-scale model was developed in this study to investigate the behavior of nanoparticle transport in tissues after intratumoral infusion. It consists of a particle trajectory tracking model that considers particle-surface interactions, and a macro-scale model for fluid flow, tissue deformation, and nanoparticle transport through deformable tissues. The multi-scale model was used to understand the effects of tumor deformation and particle binding to the cell surface on the particle distribution.
The objective of the current study is to investigate the interrelated mechanisms of nunderstanding of nanoparticle deposition behavior during a infusion process. anofluid transport in tumors using a multi-scale approach to achieve advanced understanding of particle deposition behavior.
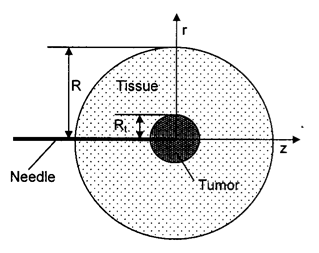
The multi-scale model was used to study infusion of nanofluid through a needle into a spherical tumor embedded in 20 mm thick normal tissue as schematically illustrated in the figure. The boundary conditions used in this study are as follows: a finite pressure and constant concentration of the nanofluid are applied at the needle tip; at the boundary, the pressure is assumed to be zero. The value of pressure at the tip is adjusted in the simulation so that it yields the desired infusion rate. No-slip conditions are applied to the surfaces of the tissue and the needle exposed to the backflow.
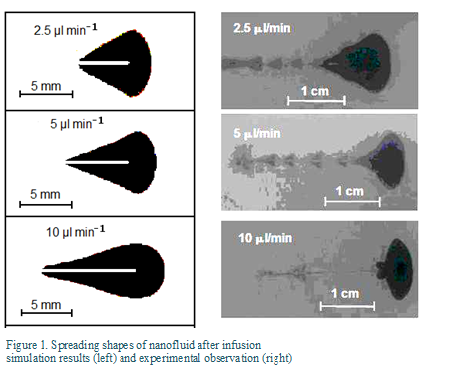
The multi-scale model was used to study infusion of nanofluid in tumors and the patterns of nanoparticle spreading and deposition in the porous tissue. Figure 1 compares the simulated shapes of nanofluid spreading in the tissue for different infusion rate to those observed in the experiment. The agreement is reasonably well for infusion at low infusion rates. The cone-shaped spreading is due to the backflow along the needle track. The difference can be attributed to breakage of the gel at the high infusion rate.
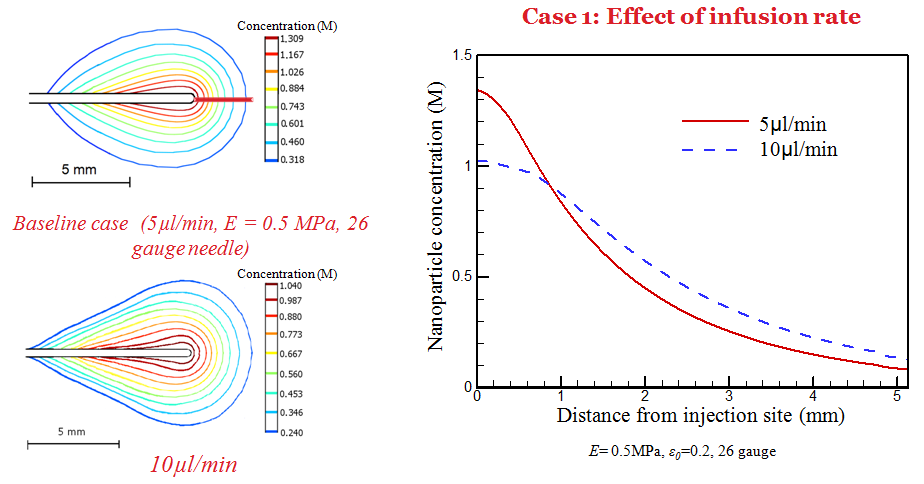
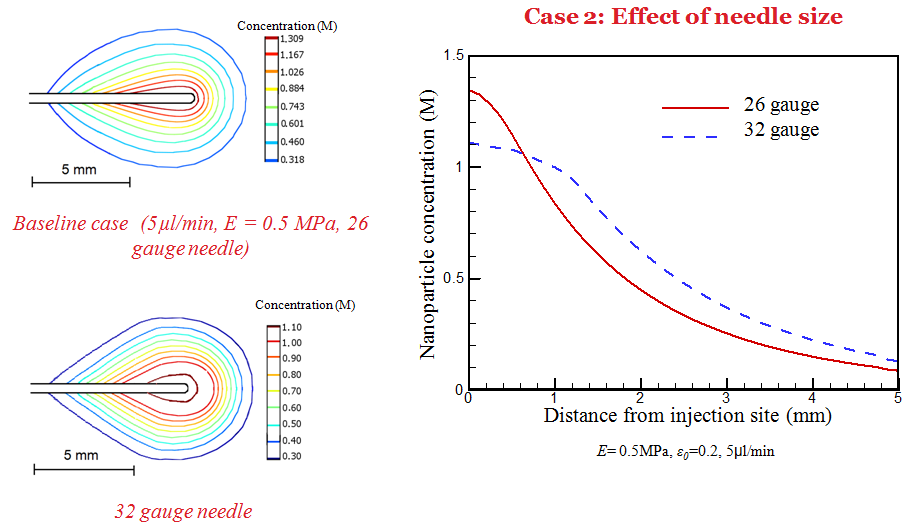
Simulation has also been performed to understand the dependence of deposition behavior on infusion, tissue properties, and needle size. It is observed that a larger needle size and low infusion rate results in a higher nanoparticle concentration near the needle tip.